Welcome
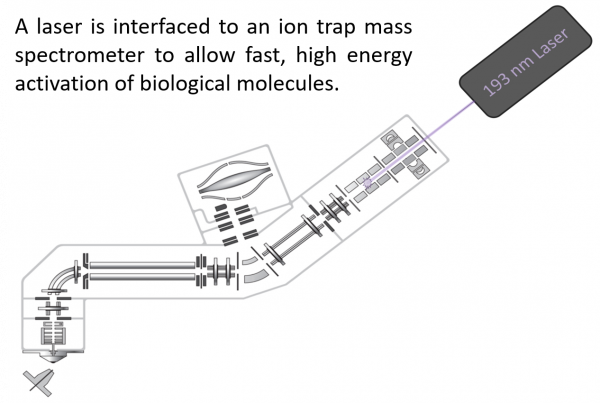
The Brodbelt group focuses on the development and application of mass spectrometry to a variety of biological problems. We are interested in developing innovative methods for activating ions to generate informative fragmentation patterns, thus improving the structural characterization of proteins, nucleic acids, and lipids. We are also implementing strategies to increase the selectivity of mass spectrometry by incorporation of chromophores or trackable mass tags that allow targeted analysis of complex mixtures.
Contact
Jennifer S. Brodbelt
Professor and Chairperson
Department of Chemistry
105 E. 24th St Stop A5300
University of Texas at Austin
Austin, TX 78712-0165
Welch Hall 3.424 (office)
jbrodbelt@cm.utexas.edu
(512)-471-0028
Research Laboratories
Located in Welch 3.102, 3.104, 3.404, and 3.410